Answer:
Option A,B,C,D
Explanation:
Plan Heat of reaction is dependent on temperature (Kirchhoff's equation) in a heterogeneous system, the equilibrium constant is independent on the molar concentration of solid species.
Heat of reaction is not affected by catalyst. It lower activation energy.
$CaCO_{3} \rightleftharpoons CaO(s)+CO_{2}(g)$
By Kirchhoff's equation
$\triangle H_2^0(at T_{2})=\triangle H_1^0(at T_{1})+\triangle C_{p}(T_{2}-T_{1})$
$\triangle H^0$ varies with temperature.
Thus, (a) ... correct
$k= p_{co_{2}}$
k is dependent on the pressure of CO2 but independent of the molar concentration of CaCO3.
Thus, (b) and (c) are correct.
At a given temperature, addition of catalyst lowers activation energy, $\triangle H$ remaining constant
Thus , (d) also correct.
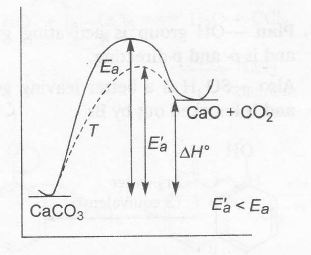
Ea = Activation energy in absence of a catalyst
Ea'= Activation energy in presence of catalyst